The Centers for Disease Control and Prevention (CDC) have commissioned a systematic review on the management of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). The review is conducted by the Pacific Northwest Evidence-Based Practice Center (EPC) at Oregon Health and Science University. A draft report was released on 17 May 2021 and interested individuals or organizations are invited to submit comments and feedback.
Much like the draft report by the National Institute for Health and Care Excellence (NICE) in the United Kingdom, the CDC review rated the strength of evidence for graded exercise therapy (GET) and cognitive-behavioral therapy (CBT) as ‘low’. The evidence for drug therapies fared no better. This blog post summarizes the main findings of the 419-pages-long document.

Introduction: the key questions
The scope and key questions used to guide the review were developed by the Pacific Northwest EPC with input from the CDC and eight key informants representing clinical, research, or patient perspectives in ME/CFS. It is unclear who the key informants are but a list will be made available in the final report. A protocol for the review is published on PROSPERO.
There are three key questions that the review tries to answer:
- Key Question 1: In patients undergoing evaluation for possible ME/CFS, what is the frequency of non-ME/CFS conditions?
- Key Question 2: What are the benefits and harms of diagnosing ME/CFS, versus non-diagnosis?
- Key Question 3: What are the benefits and harms of therapeutic interventions for patients with ME/CFS, and how do they vary by patient subgroups?
- Key Question 3a: Interventions for treating ME/CFS
- Key Question 3b: Interventions for treating symptoms commonly present in persons with ME/CFS (fatigue, poor sleep, orthostatic intolerance, pain, cognitive problems, depression, multiple chemical sensitivity, gastrointestinal symptoms, urinary symptoms, etc.)
Key Question 1: Non ME/CFS diagnoses in patients evaluated for ME/CFS
For Key Question 1, five relevant studies and one systematic review were found in the literature. The systematic review looked at the diagnoses of people presenting with fatigue in primary care. It found that depression was common (around 18.5% of cases) and that serious somatic disease was rare (approximately 4.3% of cases). Anaemia, diabetes, hypothyroidism, and malignancy were among the most frequent somatic diagnoses.
Three studies looked at the diagnosis of ME/CFS among patients presenting with tiredness in primary care. Two found a low prevalence of ME/CFS (1.9% and 0.7%) while the other found a rather high prevalence of 31.2%. This difference might have been the result of how pre-selected the samples of patients were. In the latter study, for example, patients already had unexplained fatigue for at least 6 months, an important requirement for ME/CFS diagnosis that might explain why a substantial proportion met ME/CFS criteria. Because of large differences between studies, the authors of the review preferred not to pool the data on ME/CFS prevalence into one number.
Four studies looked at patients with suspected ME/CFS who were referred to a specialist center for clinical examination. These studies found that a lot of patients (approximately half) did not have ME/CFS but another diagnosis. The draft report commissioned by the CDC states that “the proportion of patients who met criteria for ME/CFS ranged from 36% to 60%.” The big question is: what was the diagnosis of those who did not have ME/CFS? These are the conditions that physicians need to keep an eye on when they examine patients with suspected ME/CFS.
In three European studies, the most common conditions turned out to be psychiatric (15% to 51% of cases) and sleep disorders (6% to 30% of cases). A fourth, American, study was different in many respects. It invited healthcare providers and an ME/CFS support group in Georgia to refer patients for a clinical examination. In almost half of the participants, a diagnosis of ME/CFS was excluded. The most common non-ME/CFS conditions were alcohol abuse (8.2%), anemia (6.1%), diabetes mellitus (16%), high C-reactive protein (20%), hypothyroidism (20%), depression (8.2%), restless legs syndrome (6.1%), substance abuse (6.1%), and urinary tract infection (8.2%).
Key Question 2: The benefits or harms of diagnosing ME/CFS
The second question was if benefits or harms were associated with a diagnosis ME/CFS compared to non-diagnosis. Although some studies discussed this topic, none provided reliable data on the topic.
Key Question 3: Therapeutic interventions for patients with ME/CFS
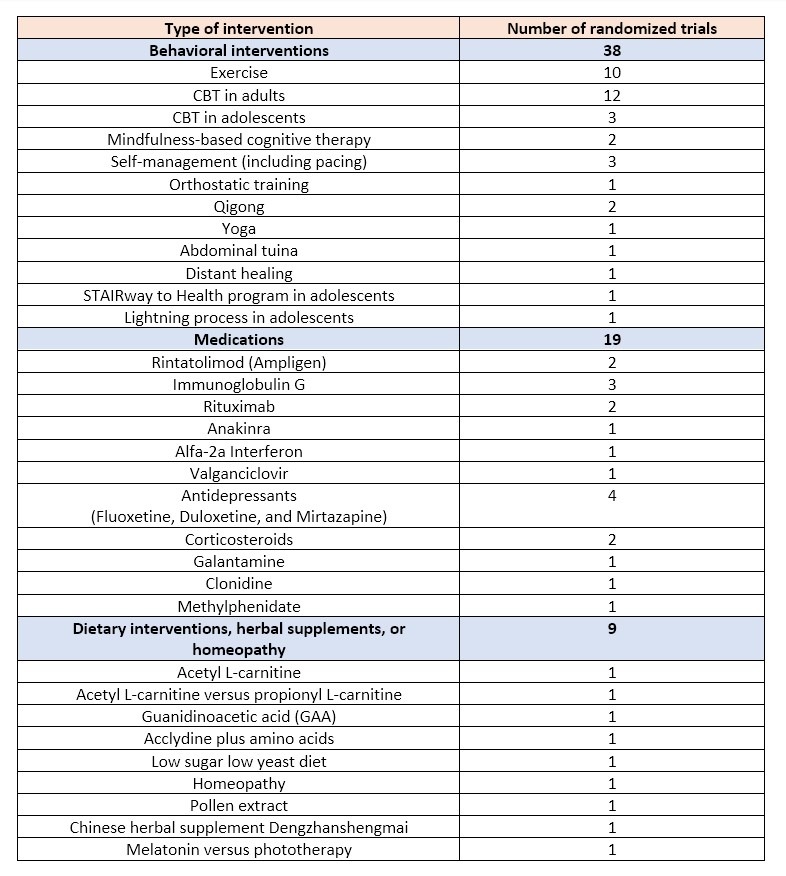
Most of the review deals with Key Question 3 on interventions for treating ME/CFS or symptoms commonly present in persons with ME/CFS. 66 relevant studies were found. As the overview below shows, the majority (38/66) of trials tested the use of behavioral interventions. There were also plenty of trials on dubious interventions such as distant healing, the ‘Lighting process’, homeopathy, the ‘STAIRway to Health’ program, pollen extracts, abdominal tuina, yoga, qigong, and various herbal supplements. Only 19 of the included randomized trials tested the use of a medication to treat the symptoms of ME/CFS patients.
Nothing but low evidence
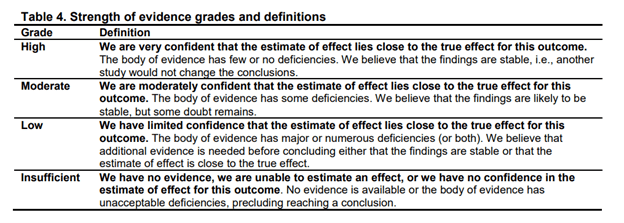
The main conclusion from the report is that there is little to no reliable evidence for any intervention. The review used the grading system formulated by the Agency for Healthcare Research and Quality (AHRQ). It consists of 4 grades: ‘high’, ‘moderate’, ‘low’, and ‘insufficient’ with explanations for each label given in the image below. For all treatments for ME/CFS, the strength of evidence was considered low or insufficient (see Table 22. Summary of Evidence, starting on page 153). This means that the reviewers have limited confidence in the estimates of effect and that the body of evidence has major or numerous deficiencies (or both).
The report states that “the strength of evidence for exercise therapy and CBT versus inactive therapies was rated low, even though these represented the most robust bodies of evidence on treatments for ME/CFS.” This is in agreement with the draft report of the NICE committee on ME/CFS in the United Kingdom, which also rated the quality of evidence for exercise therapy and CBT as low to very low.
The report commissioned by the CDC noted several limitations of the evidence supporting exercise therapy and CBT.
- It found high ‘heterogeneity’, meaning that different studies found very different effects.
- The magnitude of effect was often small. The review states that the “effects ranged from below to slightly above proposed thresholds for minimum clinically important differences.”
- Studies often used outdated case definitions of ME/CFS. Multiple trials used, for example, the Oxford case definition, which is considered less stringent than recent ME/CFS criteria and could have resulted in potential misclassification (overdiagnosis) of patients with other fatiguing conditions.
- The reviewers note that in exercise and CBT trials patients nor therapists could be blinded and that this might have biased the results. They state that “the inability to blind is of particular concern for subjective outcomes such as fatigue and function.”
I think several other limitations are overlooked in the review such as the inadequate control condition in exercise- and CBT trials. Often patients in the control group received no intervention and were put on a waiting list – not exactly the most exciting option. Little attention is paid to objective outcomes which are less affected by bias and tend to show little to no improvement. And most of the exercise and CBT trials were rated as ‘medium’ risk of bias (even the PACE and SMILE trials) while they are clearly at high risk of bias.
Unfortunately, the report also does not explain in the abstract that the strength of evidence for exercise therapy and CBT was considered low. Instead, the main conclusion is described as follows:
“Although graded exercise and CBT were more effective than inactive control therapies (usual care, usual specialist care, or an attention control) in improving fatigue, function, and other outcomes, the magnitude of effects was small to moderate and methodological and other limitations (imprecision, inconsistency, uncertain generalizability) precluded strong conclusions.”
I think that description is too ambiguous and vague and I hope it will be changed in future versions of the report. In my view, the reviewers still underestimate the risk of bias in non-blinded trials where subjective questionnaires are used as primary outcome measures, where the intervention attempts to manipulate how patients interpret their symptoms and where patients in the control group received no intervention at all. It should be made clear that those trials are at high risk of bias.
Exercise therapy: the outlier by Powell et al.
I will articulate my critique of the report more thoroughly in a future blog post. For now, let’s forget about risk of bias briefly and take a naïve look at the evidence summarized by the report. It provides some interesting insights. For exercise therapy, 10 trials were included: the 8 used in the Cochrane review, the GETSET trial (Clark et al. 2017), and a small German study by Windhorst and colleagues.
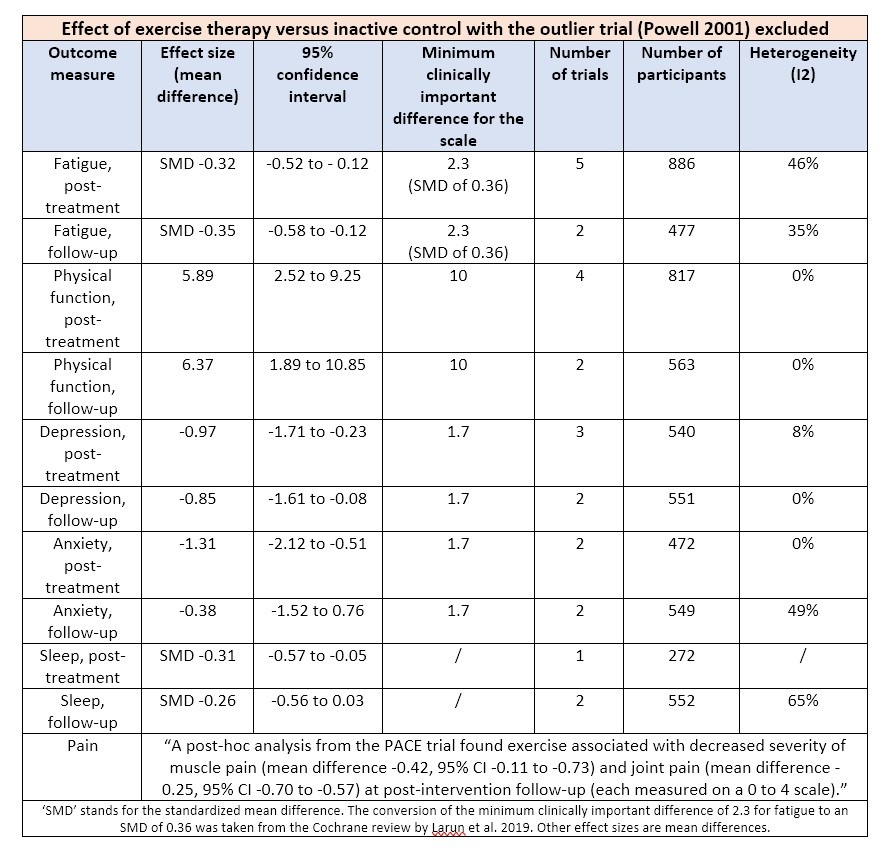
With these 10 trials, several analyses were conducted depending on the type of intervention, control condition, outcome measure, and time of assessments. The main analyses compared exercise therapy with an inactive control and included only 6 trials. These all suffered from high heterogeneity because the study by Powell et al. reported a much bigger effect size than the others.
There has been quite a lot of criticism of the PACE trial because the authors deviated from the protocol to inflate treatment effects (for a good overview of the issues see this paper). It isn’t the PACE study, however, but the trial by Powell and colleagues that stands out. It reported an effect on post-treatment fatigue that is more than 4 times larger than the effect size recorded in the FINE, PACE, and GETSET studies. Powell and colleagues published their findings in 2001 without a pre-specified protocol so that the results could not be scrutinized as has been the case with more recent studies.
If the outlier trial by Powell et al. is excluded, there is no longer a significant effect of exercise on almost all outcome measures. As the overview below shows, the mean difference found is almost always lower than the minimum clinically important difference chosen by the reviewers.
Exercise therapy was also compared with active interventions such as relaxation therapy, adaptive pacing therapy, heart rate variability biofeedback, and fluoxetine but these comparisons also failed to provide reliable evidence of effectiveness. Either the estimate of effect was not statistically or clinically significant or the results came from only one study.
CBT: the Dutch studies with a waiting list control group
12 trials evaluated CBT in adult patients with ME/CFS. When compared to an active intervention such as exercise therapy, relaxation therapy, cognitive therapy, mirtazapine, and adaptive pacing therapy, only the latter comparison showed statistically significant results in favor of CBT but the data came from just one study (the PACE trial).
Only when compared to a passive control condition such as usual care or a waiting list, does the CBT group consistently do better. The figure below shows the main result for fatigue at the end of treatment.
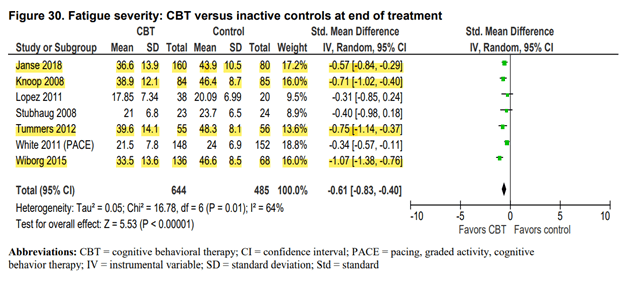
The studies highlighted in yellow are the ones that found the largest effect in favor of the CBT group. They all used a waiting list control design and they were all conducted by the same research team from the Netherlands. These researchers hypothesize that ME/CFS is maintained by deconditioning and unhelpful thoughts and behavior such as focusing on symptoms. They have stated that their version of CBT is curative and that patients are encouraged to no longer label themselves as ME/CFS patients. Perhaps this explains the large effect sizes found, as their version of CBT is more aggressive in influencing how patients view their symptoms.
Nonetheless, it is worth highlighting that there is little evidence of the effectiveness of CBT on other outcomes than fatigue (even when compared to a waiting list). If we ignore all the limitations of the studies and take results at face value, there is still no convincing evidence that CBT improves physical function, depression, anxiety, walking ability, etc. For sleep, joint- and muscle pain there was data from only one study (the PACE trial).

Harms: imprecise and poorly recorded
The review states that harms of CBT and exercise therapy were not well reported. In the case of exercise therapy, data on harms came from only two trials, namely PACE and GETSET. Although exercise therapy was not associated with serious adverse events or worsening of physical functioning, the estimates were imprecise. Unfortunately, the review did not include the multiple surveys where ME/CFS patients report that their health deteriorated following graded exercise therapy. Hopefully, this will be taken into account in the final version of the report.
Drug treatment
Next, let’s take a look at the evidence for drug interventions to treat symptoms of ME/CFS patients. There were three studies on immunoglobulin G, four on antidepressants (fluoxetine, duloxetine, and mirtazapine), and two on corticosteroids. One trial tested the antiviral valganciclovir, another study trialed alfa-2a interferon. None showed convincing evidence of effectiveness.
The drug that provided the most signs of effectiveness is Rintatolimod, better known as Ampligen. There were only two randomized trials. They showed that patients who received Ampligen performed better on an exercise test than those who received placebo but the difference was small and not seen on other measures of physical functioning. One trial also reported that adverse events such as infusion-related headache, flu-like syndrome, chills, vasodilatation, and dyspnea occurred more frequently with Ampligen than placebo. The two trials were conducted by Hemispherx, the company that patented Ampligen and has a large commercial conflict of interest in testing its efficacy. The review also notes that Ampligen was reviewed by the FDA for use in ME/CFS in 2012 but that it failed to receive approval and that no randomized trials have been conducted since.
There were also a couple of trials on supplements such as acetyl L-carnitine and melatonin but again there was little evidence of effectiveness.
Only four trials were rated at low risk of bias trials
Lastly, I decided to take a closer look at ME/CFS trials that were rated at low risk of bias in the report. Only four trials met this criterium.
The most recent one is the 2019 study on rituximab. For those who might have missed it, a few years ago Norwegian oncologists reported that ME/CFS patients improved markedly when they received cancer treatment. Subsequent investigations reported encouraging results for rituximab, a drug that targets B-lymphocytes. A large randomized trial was set up that met all quality criteria but unfortunately, it showed that rituximab didn’t work after all.
Another low risk of bias study was conducted by the research team of Jos Van Der Meer in the Netherlands and tested the use of the Interleukin-1 receptor antagonist Anakinra. Several studies have reported an abnormal pattern of cytokine activation in ME/CFS patients although the results are rather inconsistent. Interleukin-1 is one of the proinflammatory cytokines most frequently associated with fatigue. The Dutch study showed that Anakinra doesn’t improve fatigue or other outcomes in ME/CFS patients.
A third low risk of bias study was done by the Norwegian research team of Vegard Bruun Wyller. It tested the use of clonidine in adolescents with ME/CFS. The researchers speculated that ME/CFS is characterized by enhanced sympathetic nervous activity and they hoped that clonidine would inhibit this overactivation. It did, but this didn’t result in an improvement in symptoms. The authors concluded that “low-dose clonidine is not a clinically useful therapy in adolescent CFS.” They even speculated “that the autonomic and inflammatory processes that clonidine blocks may have beneficial effects.”
The fourth low risk of bias trial tested the use of ‘distant healing’. It encompasses prayer and the use of thoughts to influence other individuals without utilizing known physical means of intervention. I’m not sure how the researchers got funding and approval to set up this trial but apparently, they did. Fourteen private practices for “environmental medicine’ in Germany and Austria participated. Unsurprisingly, the study found that distant healing does not work. The interesting feature of this trial is that it included a blinded and non-blinded group so that it could estimate the effect of knowing that one is receiving the intervention. The authors write that indeed, “the expectation of improvement did improve outcome.”
It probably says a lot about the state of ME/CFS research that only four trials were rated as low risk of bias and one of them tested the use of spiritual healing. I hope that future reviews on the management of ME/CFS will have better and more interesting trials to summarize.

The #MEAction online meeting
The advocacy movement #MEAction contacted Dr. Beth Unger of the CDC. She clarified that:
“…the systematic review provided little new data and the process for guideline development would not be likely to provide an advance over what is currently available from the ME/CFS Clinician Coalition and IACFSME. We will be completing the Systematic Review and will not be proceeding with guideline development until clinical trial data becomes available.”
So it seems that the CDC has no intention of making a clinical guideline for ME/CFS based on this review. Nonetheless, it is still important that evidence is summarized correctly. #MEAction is therefore organizing a community town hall on Wednesday, June 23rd at 3pm ET on how to address the flaws in the review.
Thank you for the effort required for this analysis based on science. Unfortunately the evidence review is a political document and requires a political response. Easy for me to write from my sickbed that others should go out and do something.
The fact remains CDC and NIH are running a public relations campaign instead of a research program. They must be criticized harshly for that, using the analyses of Mr Tack and others as a solid foundation.
Obviously Dr Unger knew in advance that there was nothing new for the review to find, based on the rules they set for themselves. So another million dollars (or whatever they spent) down the drain — and still ZERO publications from the no-longer-ballyhooed CDC Multi-site Clinical Assessment of CFS — an entire DECADE after the original announcements. And of course, COVID is the perfect excuse to “indefinitely delay” the NIH intramural study until it is completely forgotten.
They are killing us and won’t stop until we greatly increase the social costs of the Policy of No Research through political and legal action. People are naturally reluctant to recognize the extent of institutional corruption. After all, we in “The West” are “civilized”, allegedly. The idea that we are ruled by sadistic psychopaths is too awful to accept. But at some point reality must be recognized. I hope it happens soon.