Cortene Inc., an American pharmaceutical company, recently published findings of a preliminary treatment trial in the scientific journal Frontiers in Systems Neuroscience. In the trial, Cortene’s experimental drug ‘CT38’, was administered to 14 patients suffering from myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). An accompanying press release claimed that the drug “achieved sustained symptom improvement” and that the trial “provides preliminary evidence of a cure for ME/CFS”. Both statements are incorrect. This blog post tries to move beyond hyperbole and figure out what the trial showed.

Background info
The study, called ‘InTime’, was conducted at the Bateman Horne Center, a center of excellence in Utah that focuses on treating ME/CFS. The drug tested was the acetate salt of CT38 which acts as a corticotropin-releasing factor receptor type 2 (CRFP2) agonist. CRFP2 regulates the release of the neurotransmitter serotonin during stress. The researchers at Cortene speculate that CRFP2 is up-regulated in ME/CFS leading to increased release of serotonin and loss of homeostasis. They think that their experimental drug can alleviate ME/CFS symptoms by downregulating CFPR2. Previous studies tested the effects of CT38 in rats while the safety of the drug was assessed in a phase I trial of 64 healthy human volunteers.
InTime acted as the next step for Cortene where CT38 was administered to 14 patients with ME/CFS. Participants were between 18 and 60 years old and met the Fukuda, Canadian, and National Academy of Medicine criteria for ME/CFS. The results were published in the scientific journal Frontiers in Systems Neuroscience on 01 September 2021. The paper is open-access, meaning that it can be read without payment or subscription. The full citation is:
Pereira et al. 2021. Acute Corticotropin-Releasing Factor Receptor Type 2 Agonism Results in Sustained Symptom Improvement in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front. Syst. Neurosci., 01 September 2021 | https://doi.org/10.3389/fnsys.2021.698240
Problems with the hypothesis
Before discussing the results of InTime, there are several issues with its underlying hypothesis that should be noted. Normally an experimental drug is trialed because it is thought to correct a pathological abnormality that has been demonstrated with empirical evidence. In the case of InTime, however, the disruption that CT38 aims to fix, namely an upregulation of CFPR2, hasn’t been demonstrated yet in ME/CFS. Therefore the trial is very much a shot in the dark, with a low chance of hitting target.
Even circumstantial evidence linking ME/CFS and upregulation of CFPR2 is scarce. In the paper, the authors focus on prior stresses and their effect on the limbic system, stating that “ME/CFS develops more in conditions of adversity.” It seems that the researchers started with a drug and then searched for an illness where it could be applicable, rather than the other way around.
There’s another issue. According to the trial’s hypothesis, the neurotransmitter serotonin plays a key role in causing the symptoms of ME/CFS. Therefore, it needs some explanation why randomized trials of Fluoxetine (Prozac) and Duloxetine, both selective serotonin reuptake inhibitors, did not find noticeable effects in ME/CFS patients. If this was indeed a central pathway, then we could expect to see some impact of drugs that influence this pathway. In addition, the symptom complex caused by high levels of serotonin, is quite different from that of ME/CFS.
Even if upregulation of CFPR2 plays a key role in ME/CFS pathology, Cortene’s argumentation of why their experimental drug would fix the problem is rather unusual. “The conventional approach would be to block the overactive pathway,” Cortene’s Chief Development Officer explained. “Instead, our counterintuitive approach seeks to overstimulate CRFR2, causing it to downregulate, without the need for chronic treatment.” In other words, the researchers speculate that brief overstimulation of an already overactive pathway will put everything back to normal. CT38 is a peptide with a half-life of 1.5 hours and patients in the trial only got infusions for 2-3 days. So even though the impact of the drug is short-lived, the authors speculated that it would somehow have long-lasting effects.
Not everyone was convinced by this explanation. Michael VanElzakker, a neuroscientist at Tufts University studying ME/CFS and post-traumatic stress disorder (PTSD) has been a vocal critic of the Cortene trial, even before the results were published. On Twitter, VanElzakker stated that “the proposed mechanism of cortene strikes me as absolute nonsense.”
The trial focused on safety, not efficacy
Let’s now take a look at the data of the InTime trial because even if the underlying hypothesis has issues, it’s the results that ultimately matter. A drug might still work even if we can’t figure out why.
The first thing that we need to clarify, however, is that InTime cannot tell us whether CT38 works or not because it was not designed to measure treatment effects. It had only 14 ME/CFS participants and no control group. Therefore we don’t know if reported improvements are due to the drug or a whole range of other possible explanations such as regression to the mean, response bias, placebo effects, the natural course of the illness, and so forth.
InTime mainly tested if CT38 could be safely administered to ME/CFS patients. Unfortunately, at the start of the trial, there were some problems. The first two patients tolerated the drug poorly with one of them having a serious adverse reaction. The authors had to increase the infusion duration and decrease the dose from 0.20 µg/kg/h to 0.03 µg/kg/h. They also tested two other dose levels to see if the effects of the drug would change accordingly.
After these changes were implemented, there were still adverse effects recorded after treatment but these were milder and difficult to differentiate from regular ME/CFS symptoms. There was, however, one patient who received the lowest dose and experienced headache, facial numbness, dyspnea, dizziness, and swollen lymph nodes afterward. In this case, the investigators decided it was best to discontinue treatment.
Results contradict claims about a cure
Next, let’s have a look at the efficacy results. Unfortunately, these can’t tell us much because the trial was small and did not include a control group. Yet, even if we take all improvements at face value, they clearly contradict claims of CT38 being a “cure” for ME/CFS.
TDSS: symptom scores
The primary outcome of the trial was a mean total daily symptom score (TDSS), averaged over 28 days. On this questionnaire, 13 symptoms were rated on a 0-5 scale, and then the scores for all symptoms were summed up to a total score that could range from 0 to 65. After treatment with CT38, the TDSS scores decreased on average by 4.3 points (95% confidence interval: 1.18-7.37). That’s not a lot, as the graph below shows. In comparison, the standard deviation before treatment was approximately 9.5 points.
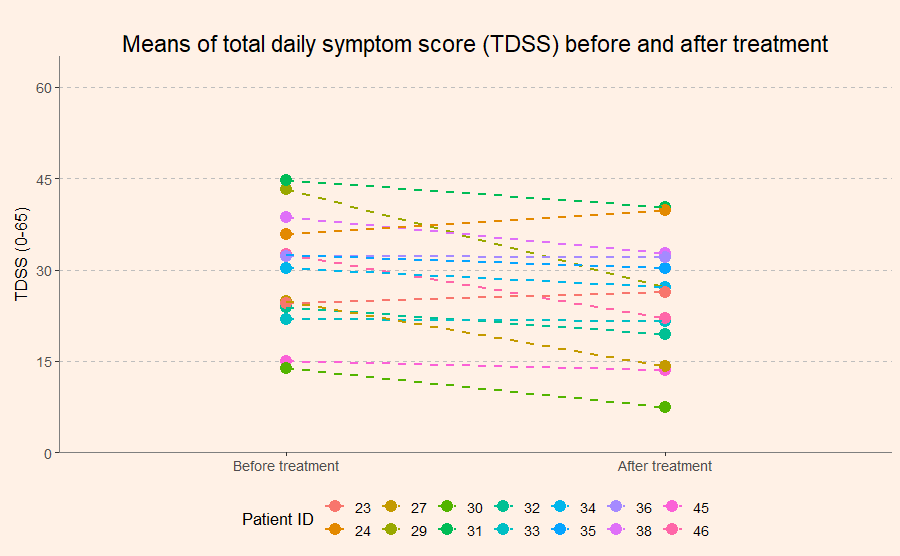
In the abstract of their paper and the press release, Cortene highlights a 25% reduction of symptom scores. This figure, however, only applies to the subgroup of 7 patients who received a dose of 0.03 µg/kg.
SF-36: physical component summary
The physical component summary of the 36-Item Short Form Survey Instrument (SF-36) was used as a secondary outcome. This scale is helpful because it is used a lot in scientific research and has been tested in various illnesses. It is an interpretable measure of how disabled a person is. Scores can range from 0 (maximum disability) to 100 (no disability). At the beginning of the trial the 14 ME/CFS patients had a mean PCS score of 27.9. This is very low. The investigators of the InTime trial highlight that these scores indicate a worse health status in ME/CFS patients than in patients with cancer, congestive heart failure or diabetes for which means of 45.1, 31.0, and 39.3 have been reported respectively. What the authors do not mention in the paper, is that the mean PCS scores after treatment with CT38 were hardly better: 31.5 in the total group and 30.7 in the subgroup that received a dose of 0.03 µg/kg.
Fitbit: Steps per day
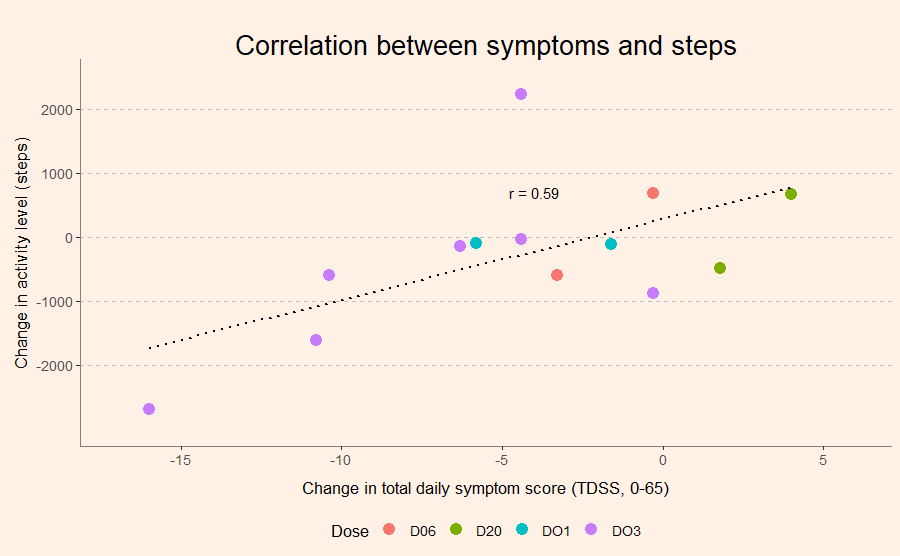
The original protocol of InTime also included daily cognitive testing and CPET, but unfortunately, these were eliminated due to practical issues. The only objective outcome was physical activity measured with a Fitbit device. Here no improvements were seen. In fact, on average, physical activity decreased after receiving CT38 in ME/CFS patients. And interestingly, the more patients reported reductions in symptoms, the more they also seemed to have reduced their activity level.
Dose-response relationship
The researchers at Cortene claim that there was a dose-response relationship, with better results in the groups that received lower doses. This is hard to tell because therapists weren’t blinded to group allocation. And while patients in the 0.03 µg/kg group reported the most improvements, the other groups had only 2 or 3 participants. An inverse dose-response relationship would also be difficult to reconcile with the trial hypothesis. Given that the original idea was to overstimulate neurons and induce endocytosis, it would be curious if this happened only in groups that received the low instead of the high dose.
One of the more interesting results of the InTime trial is that the heart rate and blood pressure of ME/CFS patients responded more strongly to the drug than those of healthy controls. This was the reason why the researchers had to adjust the dose and infusion time. There are, however, many possible explanations for this finding. It could be that ME/CFS makes people more sensitive to drugs (as has been reported previously), or that infusions take a harder toll on (mostly female) patients who are severely disabled compared to healthy (all male) controls.
Conclusion
Unfortunately, we can’t conclude much from the findings of the InTime trial except that they contradict claims about a cure for ME/CFS. Although It is great that a pharmaceutical company wants to test a new drug to treat ME/CFS, the results do not look that promising. The researchers stated that they plan to test CT38 using longer or additional infusions in future trials. We can only hope that these will show more encouraging results.
Ha finally some more Cortene-critique, from the start of this I’m like ‘wtf?’
Thanks – it is hard to parse research results when your brain is mush (like mine), but you’ve done a nice job.
Real science has balance – if they think they need a bigger trial, maybe some better results will appear. But I’m not getting excited yet – except that I’ve always felt this kind of process – leaving something in a state which needs to be reset – might hold the answer. Gut feeling – no scientific evidence. But it’s really hard to believe that something that gets into the wrong state would persist for almost 32 years for me. With small functionality increases over the years probably due to learning pacing, and decreases most probably due to aging.