In 2024, the National Institutes of Health (NIH) will fund 25 research projects on myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). The total funding amount for ME/CFS research has sadly decreased over the past few years, totalling only 10-13 million, depending on which studies you include. There are nonetheless several interesting scientific projects whose results will be eagerly awaited. A brief overview.
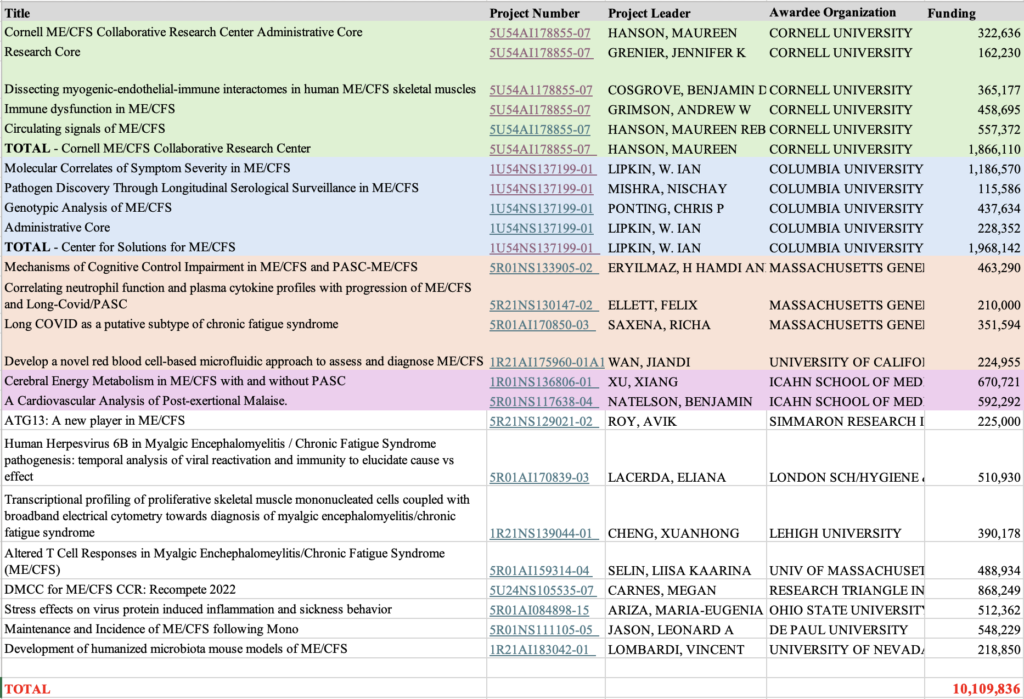
NIH funding has stagnated
The NIH in the United States has a budget of 48,000 million dollars, making it one of the biggest funders of medical research worldwide. It writes out thousands of competitive grants each year but only a handful is awarded to ME/CFS research. As the graph below shows, NIH funding for ME/CFS research has stagnated over the last 15 years.
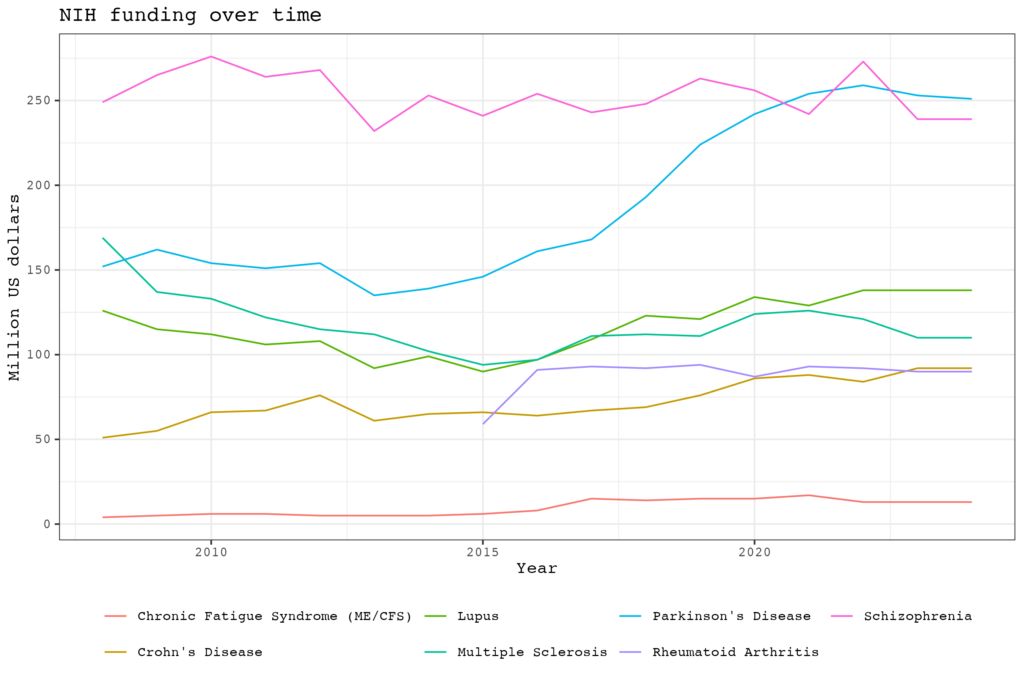
There is even a sharp decline since 2021. The NIH estimates that in 2024 $13 million of extramural grant funding will go to ME/CFS research but this may be an overestimate. In the past the NIH has included studies in the ME/CFS category that had little to do with ME/CFS or where the illness was just a small side project. When we list all the studies that focus on ME/CFS we got a total amount of 10.1 million dollars. In comparison, when we did the same exercise 4 years ago, the total sum was 11.7 million.
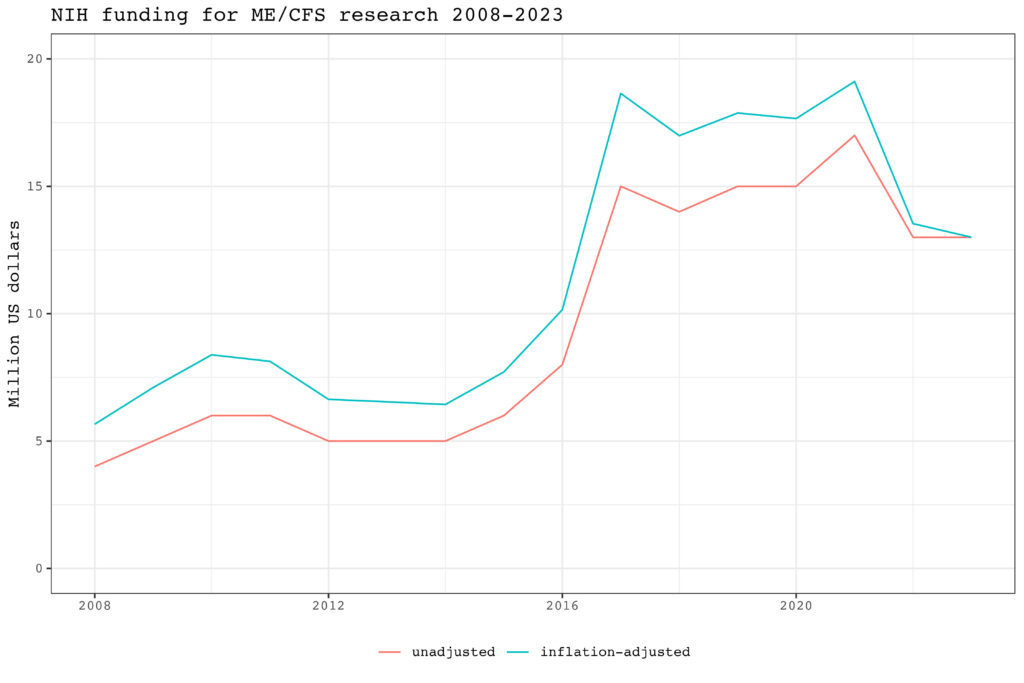
An important reason for this decline is that funding for one of the three ME/CFS collaborative research centers, the one at Jackson laboratories led by Derya Unutmaz, has not been renewed. In addition, Stanford has also not been able to get any NIH grants in 2024. Its ME/CFS research is funded through other sources such as private donations from the Open Medicine Foundation. Two collaborative research centers were able to secure funding until 2028-2029. One is led by Maureen Hanson at Cornell University, and the other by Ian Lipkin at Columbia University. They each receive just short of 2 million dollars per year, which is approximately the same amount they were getting four years ago.
Good and bad days at Columbia
At Columbia, most of the funding goes to a special longitudinal study. 60 ME/CFS patients and 60 controls will use a smartphone app to track their symptom severity and whether they are having a particularly good or bad day. Immediately after a participant enters data, the info is sent to the study server and (on good and bad days) to a researcher who will visit the participant to obtain blood, saliva, and feces, all within 24 hours. This allows researchers to study metabolomic and immunological data over time and see which markers correlate with good and bad days.
In another study, Columbia researchers will check ME/CFS patients’ past exposure to infections using the Department of Defense Serum Repository (DoDSR). Samples from people with ME/CFS, before and after diagnosis, and from matched controls will be tested using a phage display system that shows antiviral responses of ongoing or past infections.
The Columbia project also includes a genetics study led by Chris Ponting. It will use DNA samples from 5000 ME/CFS patients and compare them to 400,000 healthy controls genotyped by the Kaiser Permanente Research Bank. This dataset could act as a US comparison study for the DecodeME Study in the UK.
Cornell’s muscle biopsies
An interesting study at Cornell University will take muscle biopsies of 40 ME/CFS patients and 20 controls. The goal is to investigate interactions between endothelial, and muscle-resident immune cells using innovative single-cell technologies. Another project will examine gene regulatory changes in monocytes and abnormalities in platelets. A third Cornell study will culture endothelial cells with plasma and extracellular vesicles to see if these contain molecules that cause endothelial dysfunction in ME/CFS.
| Title | Project Number | Project Leader | Awardee Organization | Funding |
| Cornell ME/CFS Collaborative Research Center Administrative Core | 5U54AI178855-07 | HANSON, MAUREEN | CORNELL UNIVERSITY | 322,636 |
| Research Core | 5U54AI178855-07 | GRENIER, JENNIFER K | CORNELL UNIVERSITY | 162,230 |
| Dissecting myogenic-endothelial-immune interactomes in human ME/CFS skeletal muscles | 5U54A1178855-07 | COSGROVE, BENJAMIN DAVID | CORNELL UNIVERSITY | 365,177 |
| Immune dysfunction in ME/CFS | 5U54AI178855-07 | GRIMSON, ANDREW W | CORNELL UNIVERSITY | 458,695 |
| Circulating signals of ME/CFS | 5U54AI178855-07 | HANSON, MAUREEN REBECCA | CORNELL UNIVERSITY | 557,372 |
| TOTAL – Cornell ME/CFS Collaborative Research Center | 5U54AI178855-07 | HANSON, MAUREEN | CORNELL UNIVERSITY | 1,866,110 |
| Molecular Correlates of Symptom Severity in ME/CFS | 1U54NS137199-01 | LIPKIN, W. IAN | COLUMBIA UNIVERSITY HEALTH SCIENCES | 1,186,570 |
| Pathogen Discovery Through Longitudinal Serological Surveillance in ME/CFS | 1U54NS137199-01 | MISHRA, NISCHAY | COLUMBIA UNIVERSITY HEALTH SCIENCES | 115,586 |
| Genotypic Analysis of ME/CFS | 1U54NS137199-01 | PONTING, CHRIS P | COLUMBIA UNIVERSITY HEALTH SCIENCES | 437,634 |
| Administrative Core | 1U54NS137199-01 | LIPKIN, W. IAN | COLUMBIA UNIVERSITY HEALTH SCIENCES | 228,352 |
| TOTAL – Center for Solutions for ME/CFS | 1U54NS137199-01 | LIPKIN, W. IAN | COLUMBIA UNIVERSITY HEALTH SCIENCES | 1,968,142 |
| Mechanisms of Cognitive Control Impairment in ME/CFS and PASC-ME/CFS | 5R01NS133905-02 | ERYILMAZ, H HAMDI AND VANELZAKKER, MICHAEL B | MASSACHUSETTS GENERAL HOSPITAL | 463,290 |
| Correlating neutrophil function and plasma cytokine profiles with progression of ME/CFS and Long-Covid/PASC | 5R21NS130147-02 | ELLETT, FELIX | MASSACHUSETTS GENERAL HOSPITAL | 210,000 |
| Long COVID as a putative subtype of chronic fatigue syndrome | 5R01AI170850-03 | SAXENA, RICHA | MASSACHUSETTS GENERAL HOSPITAL | 351,594 |
| Develop a novel red blood cell-based microfluidic approach to assess and diagnose ME/CFS | 1R21AI175960-01A1 | WAN, JIANDI | UNIVERSITY OF CALIFORNIA AT DAVIS | 224,955 |
| Cerebral Energy Metabolism in ME/CFS with and without PASC | 1R01NS136806-01 | XU, XIANG | ICAHN SCHOOL OF MEDICINE AT MOUNT SINAI | 670,721 |
| A Cardiovascular Analysis of Post-exertional Malaise. | 5R01NS117638-04 | NATELSON, BENJAMIN | ICAHN SCHOOL OF MEDICINE AT MOUNT SINAI | 592,292 |
| ATG13: A new player in ME/CFS | 5R21NS129021-02 | ROY, AVIK | SIMMARON RESEARCH INC | 225,000 |
| Human Herpesvirus 6B in Myalgic Encephalomyelitis / Chronic Fatigue Syndrome pathogenesis: temporal analysis of viral reactivation and immunity to elucidate cause vs effect | 5R01AI170839-03 | LACERDA, ELIANA | LONDON SCH/HYGIENE & TROPICAL MEDICINE | 510,930 |
| Transcriptional profiling of proliferative skeletal muscle mononucleated cells coupled with broadband electrical cytometry towards diagnosis of myalgic encephalomyelitis/chronic fatigue syndrome | 1R21NS139044-01 | CHENG, XUANHONG | LEHIGH UNIVERSITY | 390,178 |
| Altered T Cell Responses in Myalgic Enchephalomeylitis/Chronic Fatigue Syndrome (ME/CFS) | 5R01AI159314-04 | SELIN, LIISA KAARINA | UNIV OF MASSACHUSETTS MED SCH WORCESTER | 488,934 |
| DMCC for ME/CFS CCR: Recompete 2022 | 5U24NS105535-07 | CARNES, MEGAN | RESEARCH TRIANGLE INSTITUTE | 868,249 |
| Stress effects on virus protein induced inflammation and sickness behavior | 5R01AI084898-15 | ARIZA, MARIA-EUGENIA | OHIO STATE UNIVERSITY | 512,362 |
| Maintenance and Incidence of ME/CFS following Mono | 5R01NS111105-05 | JASON, LEONARD A | DE PAUL UNIVERSITY | 548,229 |
| Development of humanized microbiota mouse models of ME/CFS | 1R21AI183042-01 | LOMBARDI, VINCENT | UNIVERSITY OF NEVADA RENO | 218,850 |
| TOTAL | 10,109,836 |
Brain fog, neutrophils, and genetics
Three grants were awarded to Massachusetts General Hospital, although it is unclear if these are connected. A project led by Michael VanElzakker and Hamdi Eryilmaz will use brain imaging, electroencephalography, and various immunological analyses. The goal is to identify inflammation markers and brain activity patterns that underlie brain fog in patients with ME/CFS and long COVID.
Another study from Massachusetts will focus on the most common immune cells in the circulation: neutrophils. The scientists led by Felix Ellett have developed a unique panel of microfluidic assays that can test neutrophil function from a drop of fresh blood. Their preliminary results suggest neutrophile function is altered in ME/CFS patients.
The third project will compare Long Covid patients with and without ME/CFS using genetic data from 740.000 samples across several biobanks. The authors state that they “will estimate the proportion of environmental and genetic factors behind CFS and Long COVID through analysis of siblings, twins, and the general population in FinnGen and UK Biobank.”
2-day exercise testing
There are also two studies at Icahn School of Medicine at Mount Sinai. One, led by Benjamin Natelson, will focus on cardiopulmonary exercise testing (CPET) on two consecutive days. In previous blog posts (here and here), we looked closer at the 2-day CPET literature and found that it left many questions unanswered. The Natelson project hopes to provide answers, for example, by linking CPET measures to the timing and severity of post-exertional malaise. It’s rather surprising that this has not been done before. Natelson and colleagues suspect that CPET abnormalities are due to loss of total blood volume. Half of the ME/CFS patients in their study with reduced blood volume will therefore get intravenous fluid before testing on the second day. The study will include 80 ME/CFS patients, 40 in the severe and 40 in the non-severe categories, and compare them to 40 healthy controls.
The other Mount Sinai study will use MRI to compare the oxygen extraction fraction, cerebral blood flow, and glucose uptake in ME/CFS patients, Long Covid patients, and healthy controls.
Mouse models
Two studies will try to create a mouse model of ME/CFS. One is led by Avik Roy at Simmaron Research and focuses on ATG13, an important protein of cellular autophagy. By depleting this protein in mice, they expect to see post-exertional malaise-like symptoms. The other project is led by Vincent Lombardi at the University of Nevada. It will transplant fecal microbes from ME/CFS patients’ stool into mice to see if this replicates ME/CFS-associated pathology.
HHV-6B and muscle stem cells
A project that only recently received NIH funding will be the first to use single-cell analysis of skeletal mononucleated cells (SMMC) which play an important role in muscle regeneration and repair. The authors will also examine the electrical signature of muscle stem cells using a new technique called broadband electrical sensing.
In the UK, Eliana Lacerda and colleagues will use 6-month longitudinal data from the ME/CFS biobank to test if Human Herpesvirus 6B activations correspond with symptom severity. Leonard Jason from DePaul University, Chicago will conduct a 5-year follow-up of his mononucleosis cohort including those who had and did not have ME/CFS at the 6 months assessment. Lisa Selin will study T-cell exhaustion and antigen-specific responses to the Epstein-Barr Virus (EBV). Lastly, Marshall Williams and Maria Eugenia Ariza, will continue their work on EBV dUTPase as they suspect that excessive release of this enzyme plays a role in ME/CFS pathology.
ME/CFS research in other countries
In Germany, the Federal Ministry of Education and Research (BMBF) recently published the results of its call for proposals on ME/CFS. Approximately € 15 million is going to 7 projects and research networks over the period 2024-2027. We have made a summary of these projects on various social media.
In the Netherlands, a biomedical ME/CFS research program started last year with a budget of 28.5 million euros. It includes two consortia: Lifelines and NMCB. A brief description of each project is available on the ZonMw website in English.
In Austria, the WE&ME Foundation and WWTIF have finished a successful grant round at the University of Vienna. A list of these projects can be found here.
In the UK, researchers are analyzing the results of DecodeME, the largest ME/CFS to date. DNA samples from more than 20.000 ME/CFS patients were collected to perform a genome-wide associations study.
In other countries, ME/CFS research is sparse. More projects can be found on the websites of ME/CFS charities such as ME Research UK, WE&ME, the ME/CFS Research Foundation, Open Medicine Foundation, Solve ME/CFS Initiative, the ME Association, and Action for ME.
What about Australia? (See EMERGE) And Canada?
In Australia there was a targeted call from the NHMRC in 2020 and in Canada there is the ICanCME network whose funding was renewed in 2024. Both are, however, relatively small in scale.
Australia:
https://www.nhmrc.gov.au/funding/targeted-calls-research/myalgic-encephalomyelitischronic-fatigue-syndrome-funding-outcomes
Canada:
https://www.icancme.ca/news/icancme-is-renewed/