As 2024 comes to a close, it is time to review the most interesting studies on myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Although more than 300 scientific papers on ME/CFS have been published, only a few provide valuable data that bring us closer to understanding the illness. In this article, we focus on a couple of interesting studies that caught our attention. If you think we’re missing an important one, feel free to post it in the comment section below.
This is the fifth year we are making this yearly overview of ME/CFS research. Previous editions can be read here: 2023, 2022, 2021, 2020.

Differences in the blood
The most interesting study of the year came from Chris Ponting’s research team at the University of Edinburgh. This project operated without formal funding, relying on researchers working overtime outside their regular hours. It has only been published as a pre-print, meaning it has not yet been peer-reviewed by experts in the field.
Ponting and colleagues used data from 1455 ME/CFS patients aged 40-69 from the UK Biobank along with several thousands of control participants. Studies of this size have rarely been conducted in the field. The team examined 61 blood markers, 251 metabolites, and 2923 proteins adjusting their analysis for sex, age, and activity level. Challenging the common belief that ‘ME/CFS is not detectable in the blood,’ their analysis revealed significant differences in over 290 markers between patients and controls.
The researchers used data on walking and activity levels and a smart statistical technique called causal mediation analysis to exclude that differences were due to deconditioning. They conclude that the “large number of replicated and diverse blood biomarkers that differentiate between ME/CFS cases and controls should now dispel any lingering perception that ME/CFS is caused by deconditioning and exercise intolerance.”
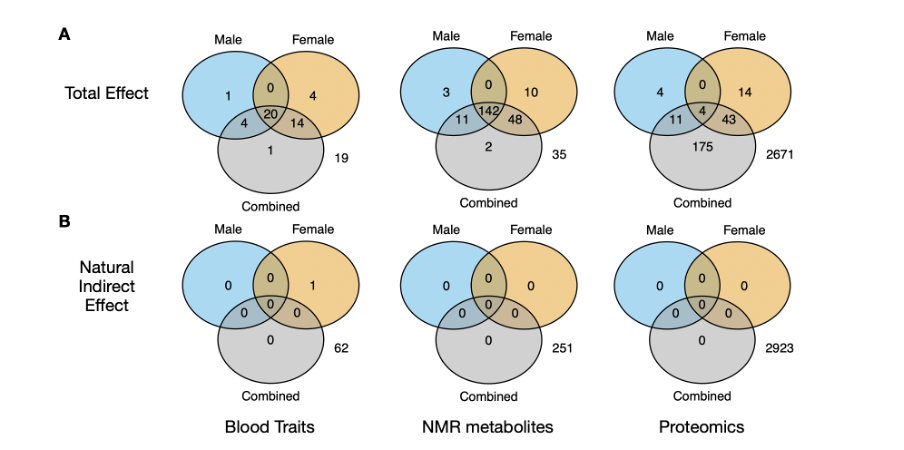
Among the abnormalities observed in ME/CFS patients, some pointed to low-grade inflammation, insulin resistance, and liver disease. The effect sizes, however, were small to modest. All blood markers showed a large overlap with controls and are unsuitable as a biomarker for the illness. And because the UK Biobank data is based on self-reported diagnoses, some abnormalities may reflect illnesses commonly mistaken for ME/CFS rather than ME/CFS itself. They might even be due to different use of medications, supplements, or diet. Despite these limitations, this study is intriguing. Among the many signals it detected, there might be clues pointing to the true underlying cause(s) of ME/CFS.
Some interesting differences were observed in the complement system and lipid profiles, areas that were highlighted in previous ME/CFS studies. The most striking results came from the proteomics analysis of male ME/CFS patients. They had increased levels of butyrylcholinesterase (BCHE), an enzyme that breaks down choline-based esters and detoxifies certain drugs. Also notable were the increased levels of leptin, a hormone that helps to regulate satiety and energy balance. Increased leptin levels have also been reported in other ME/CFS studies. Lastly, one protein, extracellular superoxide dismutase (SOD3), was abnormal in both male and female patients. This antioxidant enzyme protects the extracellular matrix and surrounding cells from oxidative damage. Hopefully, the Edinburgh team will investigate this further, for example, by exploring how these blood abnormalities cluster together.
The NIH intramural study
The NIH intramural study should have been the most exciting research in the field, but unfortunately, it has mostly resulted in disappointment.
Recruitment for this project started in 2016, with the NIH inviting participants to their campus in Bethesda for the most extensive set of biological measurements ever conducted in ME/CFS patients. These include muscle biopsies, cerebrospinal fluid draws, head-up tilt tests, cardiopulmonary exercise testing, plasma lipidomic and fecal microbiota analysis, polysomnography, and many other tests. Patients were also put on a diet and remained for 16 hours in a metabolic chamber to assess their energy and nutrient balance. Our immense gratitude goes to the ME/CFS patients who underwent all these demanding tests in the name of science!
Unfortunately, most of these measurements did not reveal significant differences between patients and controls, likely due to the small sample size. Due to the Sars-Cov-2 pandemic, the study recruited only 17 patients instead of the 40 that were planned. Initially, the project intended to include participants with past Lyme disease and functional movement disorders as control groups, but this never materialized.
To add to the disappointment, the paper that contains all these impressive measurements focuses on a problematic behavioral experiment on reward motivation, originally developed for people with anhedonia. The NIH authors used this test to argue that the defining feature of ME/CFS is an “alteration of effort preference.” Following several critiques from the ME/CFS community, the NIH is currently reviewing the study to determine if the methods justified the conclusion.
Despite this controversy, the intramural NIH study did provide some noteworthy findings. In the cerebrospinal fluid, ME/CFS patients had lower levels of enzymes that break down catecholamines, and there was a downregulation of tryptophan and butyrate, consistent with previous research. In blood samples, there was a relative increase in naive B cells and a decrease in switched-memory B cells. This suggested a blockage in the maturation of B cells. Additionally, 24% of ME/CFS participants also had positive antinuclear antibodies compared to just 5% of controls. Patients were also unable to maintain grip force and had a longer blood pressure recovery following the Valsava maneuver.
MCAM and cognitive functioning
The Multi-Site Clinical Assessment study of ME/CFS (MCAM) released several new papers in 2024. This project, organized by the CDC, recruited patients from seven top ME/CFS specialty clinics across the United States. One new paper compared patients across the sites to see if ME/CFS means the same thing in each clinic. It found that while the ME/CFS patient population is quite heterogeneous there are no major differences across the centers. The authors conclude that “expert clinicians are recognizing the same clinical entity, albeit one that is far from homogeneous.”
Another MCAM publication focused on cognitive assessments using the computerized CogState Brief Screening Battery (CBSB). These tests were administered at five different time points, including before and after an exercise test. A total of 261 ME/CFS patients and 165 controls participated, making this one of the biggest studies on cognitive functioning in ME/CFS. The results can be summarized as follows: there was no difference in accuracy between ME/CFS patients and controls, but information processing speed was significantly lower in the patient group. Adding a maximal exercise test did not result in further cognitive dysfunction above and beyond that provoked by the clinical visit.
Two-day exercise studies
Some researchers believe that exercise testing in ME/CFS patients should be repeated on two consecutive days to study post-exertional malaise (PEM). This approach has been proposed as an objective biomarker “to assist with the diagnosis of ME/CFS.” Unfortunately, the current data do not fully support that claim.
This year, the largest study on repeated cardiopulmonary exercise testing in ME/CFS was published, and it did not find a strong effect. Declines during the second exercise test are also present in many healthy controls and do not correlate well with functional disability. This suggests that the effects are smaller than initially thought and that the procedure struggles to accurately distinguish patients and controls.
The data, however, did show that ME/CFS patients declined more than controls on most outcomes. This is particularly true for VO2 peak and workload at the ventilatory threshold, key outcomes highlighted in previous 2-day CPET studies. For more details, we’ve written an in-depth analysis of the 2024 study along with a critical review of earlier 2-day exercise studies.
MAGENTA and FITNET trials
2024 also saw the results of two major rehabilitation trials. Both focused on children and adolescents with ME/CFS and were led by Esther Crawley at the University of Bristol.
The MAGENTA trial compared graded exercise therapy (GET) to activity management. Unfortunately, both treatments showed disappointing results. The authors conclude that “there was no evidence that GET was more effective or cost-effective than activity management, with very limited improvement in either study group.” Surprisingly, the accelerometer data revealed that patients in the GET group were actually less active after weeks of therapy compared to baseline. There was also evidence of deterioration in 33 out of 123 (27%) participants in the GET group and 20/117 (17%) participants in the activity management group. These results are striking because the Bristol team has been a proponent of these rehabilitation approaches. The MAGENTA trial stopped recruiting in 2018, so it is rather unfortunate that it took so long before these findings were published.
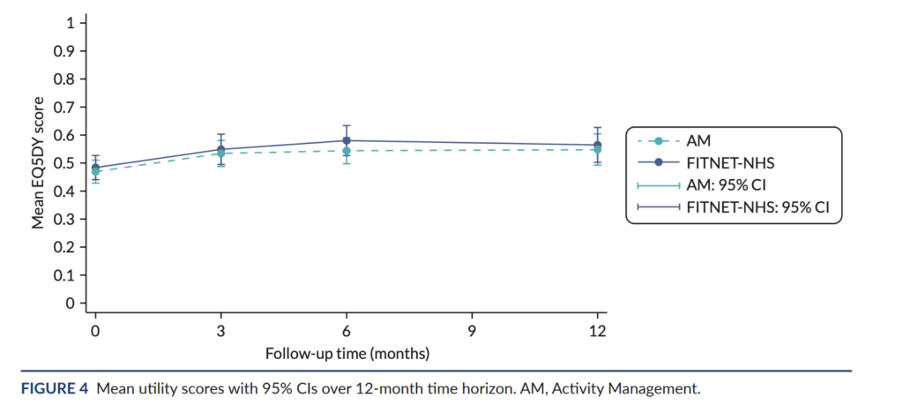
The second study, named FITNET-NHS, was an even bigger randomized trial of 314 children and adolescents with ME/CFS. It tested an online form of cognitive behavior therapy (FITNET) originally developed in the Netherlands. FITNET aims to challenge unhelpful thoughts about fatigue and encourage patients to reduce sleeping time and increase their activity levels. Dutch researchers claimed that two-thirds of children recovered with FITNET compared to only 8% in the control group. Crawley’s trial aimed to replicate these findings in the UK, comparing FITNET to an activity management control group. On the primary outcome of physical functioning, FITNET showed a slight advantage over the control group, but the difference was not clinically significant. Quality of life scores remained the same between groups across all time points and none of the statistical analyses found FITNET to be cost-effective. The authors write that FITNET-NHS “is expensive and is unlikely to be good value for money.” We wrote a detailed analysis of this trial, which a BBC article once described as “a landmark chronic fatigue trial.” You can read our full analysis here.
Prevalence studies in the UK, US and Canada
We also have a new prevalence estimate for ME/CFS. Chris Ponting and colleagues used Hospital Episode Statistics data for NHS Hospitals in England. The ICD-10 code G93.3 (“Postviral fatigue syndrome”) was used to estimate how common ME/CFS is. Between 1989 and 2023, 100,055 people received such a code in their electronic health records across 42 NHS England Integrated Care Boards. This gave a prevalence of approximately 0.16% (0.25% for females and 0.065% for males), meaning that every GP practice would be expected to have four or more ME/CFS patients registered.
However, there was considerable regional variation, likely reflecting underdiagnosis and inconsistent recognition of ME/CFS. When Ponting and colleagues used the highest ME/CFS prevalence, as recorded, for NHS Cornwall and the Isles of Scilly, the prevalence would be approximately 0.585% (0.92% for females and 0.25% for males). If we extrapolate this to the entire UK, it would suggest that 390.000 people could receive an ME/CFS diagnosis in their lifetime. This is substantially higher than the 250.000 figure that is often used today.
Other studies examined the effect of COVID-19 on the prevalence of ME/CFS. INSPIRE, a multisite, longitudinal study, included 4378 adults with acute symptoms suggestive of SARS-CoV-2 infection who underwent testing. Researchers used a questionnaire to estimate the prevalence of ME/CFS-like illness up to 12 months after infection. Unexpectedly, ME/CFS-like illness was similar in individuals who tested positive for COVID-19 (2.8%-3.7%) and those who tested negative (3.1%-4.5%). This suggests that COVID-19 is no more likely than other acute infections to be associated with ME/CFS, a finding that is supported by other studies as well (examples here, and here).
The STOP ME/CFS project had similar findings. This project uses the Kaiser Permanente Northern California (KPNC) integrated health system to track ME/CFS. A sample of 9,825 adult members were sent a survey which included questions based on the 2015 IOM-criteria for ME/CFS. The results showed that 1.7% had an ME/CFS-like illness. COVID-19 did not substantially increase ME/CFS-like illness in the KPNC population during the study time. Only 2.2% of the identified participants with ME/CFS-like illness, had an ME/CFS diagnosis in their electronic health records. This could mean that ME/CFS is highly underdiagnosed or that ‘ME/CFS-like illness’ is not a good proxy for ME/CFS.
It has previously been noted that the prevalence of self-reported ME/CFS (usually above 1%) is substantially higher than estimates from epidemiological studies (usually below 1%, with many cases remaining undiagnosed). A Canadian study by Luis Nacul and colleagues offers new insight into this apparent contradiction. They used data from CanPath, a population-based cohort data of over 330,000 Canadians aged between 30 and 74 years. 1.1% self-reported ME/CFS, but when additional questionnaires were added to ascertain ME/CFS diagnosis, the prevalence dropped to 0.4%. The authors conclude that:
“the use of self-reported diagnosis of ME/CFS is not a reliable indication to estimate the prevalence of this disease and is not well suited for clinical or epidemiological studies, due to the potential for bias as a result of misclassification of disease status.”
Unfortunately, this Canadian study had its own limitations: the sample size of ME/CFS cases was small, the response rate was low (43%), and the authors did not use a clinical examination as ME/CFS diagnostic criteria require. But if the findings hold true, it would have important implications, for example, for the recent CDC estimate of 1.3%, which is also based on household surveys and self-reported diagnoses of ME/CFS.
Wages before and after ME/CFS
A Norwegian study by Anne Kielland and her colleague Jing Liu also deserves mention for its unique focus on wage and sickness benefits before and after ME/CFS diagnosis
The authors extracted data from the Norwegian patient registers (NPR) and linked it with income, transfer, and sick leave information from Statistics Norway. Their analysis included 8485 working-age Norwegians diagnosed with the ICD code G93.3 for Postviral fatigue syndrome (which includes ME and CFS) between 2009 and 2018. The authors could compare wages in the years before and after diagnosis to a control group (represented by a dashed line in the graph below). The difference is quite dramatic. At the time of diagnosis, most of the income decline (and increase in sick leave benefits and transfers) had already occurred. After a couple of years, it reaches very low levels. Only three of the 733 persons who made less than 100,000 Norwegian Krone (approximately 10.000 euro) earned wages corresponding to a median wage 3 years later.
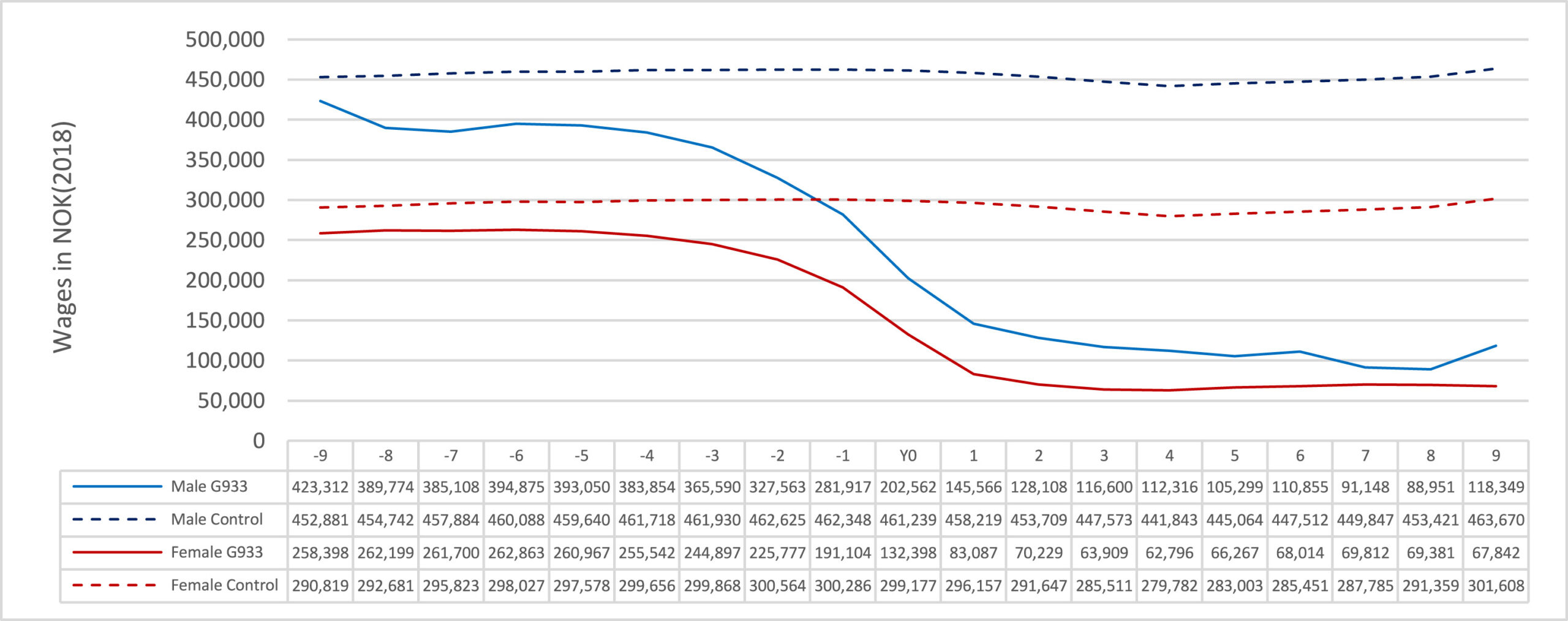
The study also included 415 people with a G93.3 diagnosis who had registered data on following a rehabilitation program. Their wages kept falling one year after the program (they had been falling for several years before that) and stabilized afterward. This suggests that the program was not very effective in getting these people back to work. Kielland and Li therefore concluded that: “health and welfare providers must address this group with realism.” They warn that:
“going into treatment and rehabilitation interventions with the explicit goal of fully recovering labor capabilities may incite false hope in people often desperate to return to their former lives.”
Long Covid
2024 brought numerous fascinating studies on Long Covid, covering topics such as ‘microclots’, cognitive slowing, T-cell activation, the complement system, and viral persistence. We picked out two Dutch studies because the authors will try to replicate their findings in ME/CFS as part of the ZonMw-funded research program in the Netherlands.
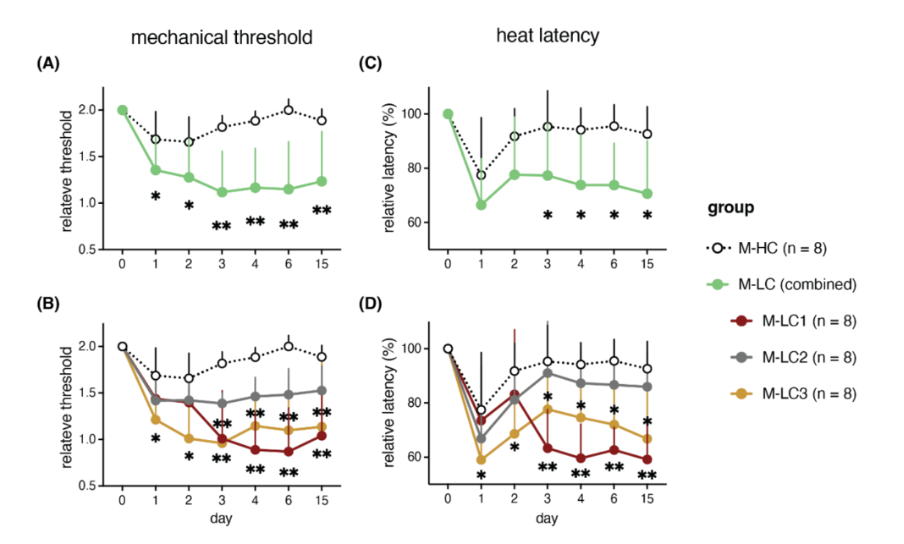
The first study by the research team of Rob Wüst took muscle biopsies of 25 ME/CFS patients and 21 healthy controls before and after an exercise test. While many amino acid levels showed no significant differences at rest, they tended to be lower in patients after the onset of post-exertional malaise. The authors also found more tissue damage and macrophage infiltration in the muscle of Long Covid patients compared to controls.
The most striking result, however, was amyloid-containing deposits in the skeletal muscle of patients. In contrast to previous reports on blood clots in Long Covid, these deposits were not located in the blood or lymphatic vessels, but in the extracellular matrix between muscle fibers. There were no signs of tissue hypoxia. Wüst’s team has received funding to do a similar study on ME/CFS patients. On X, he announced that they are “now seeing if something similar happens in patients with ME, and hope to include muscle biopsies from patients with severe ME as well in the near future.”
The second study was done by the research team of Niels Eijkelkamp and Jeroen den Dunnen who investigated the immune function of 34 Long Covid patients and 15 healthy controls. Notable differences in Glial Fibrillary Acidic Protein (GFAP) and type-I interferon levels led the researchers to use these two immune markers to classify Long Covid patients into subgroups. They then extracted autoantibodies (IgG) from each subgroup and injected them into mice to see if these induced symptoms. This seemed to be the case: mice receiving autoantibodies from Long COVID patients had a reduced sensory threshold. They felt more pain than mice that received antibodies from control participants. Similar findings have been reported by David Putrino and Akiko Iwasaki’s research team. Den Dunnen’s team has received funding to do the same tests with autoantibodies of ME/CFS patients as part of the AutonoME project.
Big surveys
In 2024, there were also several big surveys with interesting results.
- Muirhead and colleagues, for example, tested the quality of life in a large sample of 876 ME/CFS patients. The mean overall health status on a visual analog scale was 36.4 (with 100 being best health). ME/CFS patients were most impacted by the inability to perform usual activities. Anxiety and depression appeared the least affected areas.
- Schimmerl et al. surveyed 251 medical students in Germany. Approximately half disagreed that they had heard or read about ME/CFS, and 62.8% disagreed with the statement that in the absence of objective evidence for an illness, the cause of the complaints must be psychological.
- Martha Eckey and colleagues conducted a large online survey of 2,125 patients with ME/CFS and 1,800 with Long COVID on what interventions they find useful or harmful. Graded exercise therapy (GET) received the worst score with more than 80% of respondents reporting negative experiences with this intervention. This is in line with previous surveys from ME/CFS charities and with the preliminary results from the Decode ME study.
Honorable mention
Lastly, there are two papers published by Professor Emeritus Jonathan Edwards, one of the most respected voices in the ME/CFS field. Following the tragic death of Maeve Boothby O’Neill, Dr. Edwards wrote a document on managing nutritional failure in people with severe ME/CFS, including some suggestions that might supplement the NICE Guideline. Hopefully, this will help to prevent the mismanagement of patients with severe ME/CFS.
His second paper discusses the concept of ME/CFS. Although it is mainly aimed at physicians and researchers, it is also an interesting read for patients as it clarifies some misunderstandings of the history and naming of the disease.
Happy holidays
That’s a wrap! A heartfelt thank you to everyone at the Science for ME forum for their insightful discussions and thorough analyses of research papers, which were invaluable in putting together this yearly review.
If we missed any significant ME/CFS studies that you think should have been included in our review, feel free to share them in the comments below.
Wishing you all Happy Holidays and a wonderful 2025!
Thank you for the detailed analysis.
I’m unhappy to see ANYTHING with Crawley’s name on it included anywhere legitimate research is evaluated – their group’s insistence on ignoring the NICE guidelines, and all proper statistical techniques (as reviewed by many, including Dr. David Tuller), makes it a sick joke that they are still trying to justify their ‘results.’
I still see no cause, treatment, or cure in all this spending of research funds, either for ME/CFS or for Long Covid. It baffles me.