2023 will be remembered as the year in which artificial intelligence made its big breakthrough, but what did it bring for ME/CFS research? As the year is nearing its final chapter, it is time to review the most interesting ME/CFS studies of 2023.

WASF3 and the NIH’s muscle biopsies
We will start with the intriguing findings published by Paul Hwang, an NIH researcher who stumbled upon ME/CFS largely by accident.
Hwang was researching an entirely different illness, a genetic cancer called Li-Fraumeni Syndrome (LFS). His lab had shown that the mitochondria of LFS patients produce too much energy which cancer cells use to their advantage to multiply.
An LFS patient named Amanda Twinam then wrote to Hwang explaining that she suffered from disabling chronic fatigue that did not easily fit his picture of overactive mitochondria. Her symptoms were similar to ME/CFS. As Hwang’s study explains: “She reported cramps in her lower limb muscles, similar to that associated with strenuous exercise but occurring at rest, and exercise intolerance that required days to recover after physical exertion.” Amanda wondered if she had a different type of mutation that might explain her long-term fatigue.
Hwang was intrigued and decided to compare Amanda with her male sibling who had the LFS mutation but without chronic fatigue or exercise intolerance. During one of the examinations, he let both siblings perform a simple foot exercise. With magnetic resonance spectroscopy, Hwang was able to show that Amanda’s cells suffered from a slow phosphocreatine recovery after the exercise test. Phosphocreatine is a compound that plays a key role in supplying energy during short bursts of intense activity. The test therefore suggested that something was abnormal in Amanda’s muscles’ energy supply. Interestingly, a couple of old ME/CFS studies that were performed in the early 1990s, had reported similar findings. Thus, the first link with ME/CFS was made.
Hwang continued his experiments to find out what might have caused the phosphocreatine abnormality in Amanda’s muscle cells. Eventually, he stumbled upon a protein called WASF3 (Wiskott-Aldrich syndrome protein family member 3) as the potential culprit. Amanda had much more of this protein in her cells than her male sibling. WASF3 is known to play a role in various cell functions such as the organization of the cytoskeleton but Hwang suspected that it can also disrupt mitochondrial function.
He proved this with two remarkable experiments. First, he blocked the expression of WASF3 in cultured cells of Amanda and found that this restored mitochondrial function. Second, Hwang’s team developed lab mice in whom WASF3 was overexpressed, and this led to reduced exercise capacity and disrupted mitochondrial function. And there is even more to say about this protein. Interestingly, a 2011 meta-analysis had already highlighted WASF3 as a top candidate gene associated with ME/CFS, creating a second connection to ME/CFS.
It is the third link, however, that is the most interesting one. Hwang got in touch with the researchers of the intramural NIH study on ME/CFS. They agreed to collaborate and test WASF3 in the muscle samples they collected. Hwang and colleagues found that WASF3 concentrations were approximately 40% higher in the 14 ME/CFS patients compared to the 10 controls.
Because the sample sizes were small, we should not overstate this finding. Hwang also cautioned that he does not believe WASF3 to be the root cause of ME/CFS. He suspects it’s just “one of the factors mediating the energy deficiency in muscle.” He wants to dig deeper and already has a clue about what might be causing WASF3 overexpression: endoplasmic reticulum (ER) stress.
The ER is an extended network of membranes inside the cell that acts like a busy factory in which proteins are made, folded, and prepared for various tasks. When that factory is put under stress, it tends to make errors. Hwang thinks that overexpression of WASF3 might be one of these errors. His team is now looking for drugs that can reduce both ER stress and WASF3 concentrations. Hopefully, we’ll hear more about this exciting line of research in 2024.

Questionnaire studies
The first DecodeME results
2023 also saw the first results of the DecodeME study, the biggest ME/CFS study the world has ever seen. These first results do not include any of the genetic analyses yet but are based on rich questionnaire data that participants completed when entering the study. Because the study is so large – it includes data from more than 17,000 ME/CFS patients! – it is worth having a closer look at its findings.
Two-thirds of participants reported an infectious onset and for most participants, the illness onset occurred somewhere between 25 and 50 years of age. Being female, being older, and having the illness for more than 10 years, were all associated with greater illness severity.
The thing that stood out most was the strong female predominance: 83.5% of all participants were female. Although these figures may have been skewed by various biases (females may, for example, be more likely to be diagnosed with ME/CFS than males), it is quite remarkable that the difference was so large. There were 5 times as many female as male participants. In comparison, a recent review on chronic fatigue showed a much weaker female predominance where only 58% of patients were female. The high number of female ME/CFS patients has also been found in several prevalence studies so it might be telling us something important about the pathology of ME/CFS.
The main limitation of the DecodeME is that participants self-reported having an ME/CFS diagnosis made by a healthcare professional. The study did not include a clinical examination as most ME/CFS diagnostic criteria require. That was a deliberate choice because otherwise, it would have been impossible to reach the enormous sample size needed for a genome-wide association study.
The new CDC prevalence estimate
Two other studies used a similar approach: because they did not require clinical confirmation of ME/CFS diagnoses they were able to reach an enormous number of participants.
The first one was published only a couple of weeks ago by the Centers for Disease Control and Prevention (CDC) in the US. It consists of a representative household survey of the American population with more than 50,000 participants. Among the questions were: “Have you ever been told by a doctor or other health professional that you had Chronic Fatigue Syndrome (CFS) or Myalgic Encephalomyelitis (ME)? and “Do you still have Chronic Fatigue Syndrome (CFS) or ME?” 1.3 percent said yes to both questions.
This estimate is similar to Canadian census data from 2014 but much higher than what prevalence studies by the CDC had found. These older studies did require a clinical examination and found that only 0.2 to 0.4 percent of the population suffered from ME/CFS and that the majority of these patients were undiagnosed.
This difference is so large that it can hardly be explained by increased recognition of ME/CFS, changes in diagnostic practices, or the COVID-19 pandemic. It seems more likely that something has gone wrong with the survey. We suspect that confusion surrounding the term ‘chronic fatigue syndrome’ may have distorted the results of questionnaire studies. Participants with fatigue problems other than ME/CFS (burnout, sleep disorder, idiopathic fatigue, etc.) might have responded ‘yes’ to the question if they had been told they have CFS or ME. The age distribution of the CDC survey hints towards that explanation. It shows that ME/CFS prevalence was highest in the group aged 60-69 which is at odds with previous epidemiological studies.
Hopefully, further research will clear out this discrepancy. An interesting follow-up study would be to test how many of the 1.3 percent identified in the survey, meet ME/CFS diagnostic criteria after a full clinical examination.
The nurses study
In another large study published in 2023, researchers contacted more than 40,000 nurses for an email questionnaire. 102 (0.2%) of the nurses met the Fukuda criteria for ME/CFS, while another 522 (1.2%) had chronic fatigue but without additional symptoms. The most interesting result of this study was that increasing age, BMI, smoking, alcohol intake, etc. were all significant predictors for severe fatigue but not for the ME/CFS group.

Natural killer cells
Two 2023 studies were important for their null results.
The first one looked at Natural Killer (NK) cells and their ability to damage and destroy other cells; their so-called ‘cytotoxicity’. In the past, reduced NK cytotoxicity was often thought to be one of the few objective abnormalities in the ME/CFS field. The 2015 Institute of Medicine report on ME/CFS, for example, called it “one of the most consistent findings in ME/CFS subjects”. In more recent years, however, there have been a few studies that failed to find reduced NK cell function in ME/CFS patients compared to healthy controls. In 2023, a big one was published that could not find any difference either.
This was the Multi-Site Clinical Assessment (MCAM) study organized by the CDC. The idea behind the MCAM study was to enroll participants at multiple expert ME/CFS clinics in the US and subject them to the same protocol of tests and measurements. This allows for bigger sample sizes and more precise estimates. A previous MCAM publication was able to dispel various misconceptions about exercise testing results in ME/CFS (see our 2022 review for a discussion of these findings). The results on NK cytotoxicity would also bring remarkable results.
174 ME/CFS patients were recruited at 5 specialty clinics: the Mount Sinai clinic of Benjamin Natelson in New York, the Institute for NeuroImmune Medicine by Nancy Klimas in Miami, the Bateman Horne Center by Lucinda Bateman in Utah, The Open Medicine Clinic of Richard Podell in California and Sierra Internal Medicine by Daniel Peterson in Incline Village, Nevada.
When the NK cell function of patients was compared to those of healthy controls, their values were almost exactly the same. The researchers also looked for subgroups such as patients with severe ME/CFS or patients with a sudden onset of ME/CFS, but these also had normal NK cell cytotoxicity. There was also no significant correlation between NK function and various symptom questionnaires.
We don’t know if that is the last word in the story on NK cell function in ME/CFS, but it does show that we cannot assume this to be an established finding. Some studies, such as the ones conducted at Griffith University in Australia, were already focused on trying to explain abnormal NK cell function in ME/CFS. The MCAM study suggests we should take a step back and try to lay stronger foundations first.
The end of the virus hunt?
The other important null result came from the team of Ian Lipkin at Columbia University. Lipkin is one of the most renowned virus experts in the world and is frequently consulted by governments and media outlets on the topic.
In his 2023 ME/CFS study, Lipkin assembled one of the most thorough screenings for virus particles in ME/CFS history. His team used two large cohorts of more than 100 ME/CFS patients to study their blood, feces, and saliva. They used various complex screening methods (MassTag PCR, VirCapSeq, Ion Torrent Proton platform screening, etc.) but still, they could not find any notable difference between ME/CFS patients and healthy controls.
The only exception was a lower prevalence of anelloviruses in patients compared to healthy controls. Anelloviruses are common in humans and often do not cause any symptoms. Lipkin and his team speculate that their lower prevalence in ME/CFS may indicate a hyperimmune status, but it could also be a consequence of antiviral drugs as patients who took antivirals were not excluded in this study.
Lipkin and his team did not test for viruses in tissue such as muscle samples. But as someone pointed out to us: “The question is what these viruses can do while replicating at such a low frequency that they are not detected in the blood.” Lipkin and colleagues appear to think it is time to move forward, writing: “Our findings suggest that future investigations into viral infections in ME/CFS should focus on adaptive immune responses rather than surveillance for viral gene products.”
Fibronectin and the herpesviruses
But there is more to say about viruses and ME/CFS. Another line of research has focused on the reactivation of common herpesviruses and how these can disrupt energy production and immune function in ME/CFS. Virologist Bhupesh Prusty is one of the most prominent proponents of this view and an expert in the field. His paper on the human herpesvirus 6A (HHV-6) from last year was published in the prestigious journal Nature. Prusty suspects that herpesviruses such as HHV-6 may be playing an important role in the pathology of ME/CFS.
His 2023 preprint provides further evidence for this hypothesis. Like previous papers by Prusty, however, it consists of a series of complex experiments that are sometimes difficult to follow. We will try to break them down into two parts.
First Prusty and colleagues looked at antibodies against herpesvirus-dUTPases: enzymes that viruses make when they reproduce. ME/CFS and Long Covid patients had more antibodies against these enzymes of the Herpes Simplex Virus, HHV-6, and the Epstein-Barr Virus. In a further experiment, Prusty provided evidence that these dUTPase enzymes can alter the architecture and function of mitochondria.
Second Prusty and his team looked more closely at the antibodies of ME/CFS patients and the proteins they bind with. ME/CFS patients tended to have fewer antibodies bound with the protein fibronectin (FN1) than healthy controls. To investigate this further Prusty’s team measured FN1 levels in the blood and found that these were higher in ME/CFS patients compared to controls. So far, the evidence pointed towards something interesting. But when Prusty’s lab set up a bigger test of the antibodies against FN1 using ELISA assays, unfortunately, there was no longer a significant difference between ME/CFS patients and healthy controls. Only in the subgroup of severe ME/CFS was there a reduction of antibodies against FN1. For Long Covid patients the results were more complex: the antibodies against FN1 were reduced in all SARS CoV-2 positive patients even those without Long Covid, but the more symptomatic participants were, the lower their values tended to be.
According to Prusty and colleagues, these findings suggest FN-1 may be used as a “biomarker for the severity of both ME/CFS and long COVID” with “an immediate implication in diagnostics and development of treatment modalities.” We do not think that the evidence is strong enough yet to support these statements. Nonetheless, a connection with FN-1 would be interesting as the protein plays a role in various cellular activities including tissue repair and cell migration.

Raman Spectroscopy
Another paper that received quite a lot of attention was published by the research team of Karl Morten at Oxford University. They used a new technique called ‘Raman spectroscopy’ which shines light on molecules to then measure the resulting scattering and refracting of that light. Because of its chemical composition, each type of molecule will interact with the light differently, causing a unique pattern much like a fingerprint. By analyzing changes in the scattered light, the Oxford researchers could identify which molecules were present in a single cell. They did this in 61 participants with ME/CFS, 21 with Multiple Sclerosis (MS), and 16 healthy controls.
These Raman spectroscopy measurements gave them a wealth of data. Among the things they found were an increase in tryptophan and tyrosine, elevated glycerol levels, reduced cholesterol, and reduced glycogen levels. To make use of all the data, Morten and his team employed a machine learning algorithm that, after some training, was able to distinguish between ME/CFS patients, healthy individuals, and MS patients with a high accuracy rate of 91%. In addition, it was able to differentiate mild, moderate, and severe ME/CFS patients with 84% accuracy.
These are interesting findings. One big caveat, however, is that a wealth of data can easily create spurious relationships. Further testing is needed to see if these ‘Raman profiles’ have the same accuracy in other samples. The British ME Association announced that it will fund further work of Morten and colleagues to see if a cell-based diagnostic test for ME/CFS can be developed using Raman Spectroscopy data.
Provocation studies
Cognitive testing after standing
Three studies investigated ME/CFS patients after an exercise or provocation test in the hope that this would provide more clues into the underlying pathologies of the syndrome.
The first study was conducted at the Bateman Horne Center and consisted of a brief cognitive test before and after an orthostatic challenge. The cognitive test was performed on participants’ smartphones and mostly measured reaction time and attention. The orthostatic challenge was the Lean Test where participants needed to stand upright for 10 minutes straight. 34 Long Covid patients, 140 ME/CFS patients, and 82 healthy controls were recruited into the study.
The results were interesting. While the scores of the healthy controls improved shortly after the orthostatic challenge, those in the ME/CFS and Long Covid groups declined. The authors also looked if there was a correlation between hemodynamic variables such as heart rate or pulse pressure and cognitive performances to see if one could explain the other, but the relationship was complicated. For ME/CFS patients who had been ill for more than 10 years, for example, there was no significant correlation between hemodynamic changes and cognitive impairment.
From the gut to the bloodstream
The second provocation study came from Columbia University and focused on the gut. The researchers first used a large database of ME/CFS samples to test their hypothesis of ‘microbial translocation’. This hypothesis assumes that microbes from the gut migrate into the bloodstream where they don’t belong and consequently get attacked by the immune system.
The Columbia researchers suspected that this might be the case in ME/CFS patients and published several findings that support this view. They found, for example, elevated levels of Fatty Acid-Binding Protein 2 (FABP2), a marker of intestinal epithelial cell damage, suggesting the gut is less able to prevent bacteria from entering the bloodstream. Compared to healthy controls, ME/CFS patients also had higher levels of antibodies against proteins found in the tails and outer membranes of bacteria and more antibodies against dietary proteins, such as gliadin (a component of gluten) and casein (a milk protein).
There was, however, one thing that did not fit. The immune cells that should be busy cleaning up these gut microbes, LBP (Lipopolysaccharide-Binding Protein) and sCD14 (soluble Cluster of Differentiation 14), were not elevated in ME/CFS patients. The researchers therefore suspected that something was defective in the patients’ immune response.
Because exercise can disrupt the gut mucosal barrier in healthy adults, the researchers thought that they could use an exercise challenge to put the immune response of ME/CFS patients to the test. Unfortunately, the study was only able to recruit 9 ME/CFS patients and 7 healthy controls, and the proportion of females was much lower in the control group. The results were nonetheless interesting.
After the exercise test, LBP and sCD14 increased in the control group but not or much less so in the ME/CFS group. On the other hand, antibodies against microbe fragments increased after exercise in ME/CFS patients but not or much less so in the control group.
The immune system of ME/CFS patients appeared to be working differently. The acute immune response was missing while the humoral immune response (the one that comes later and involves antibodies) was working harder. The researchers speculate that this increased antibody response could be a compensatory mechanism to address the insufficient acute immune response by LBP and sCD14.
Urine metabolomics
The third provocation study came from Cornell University. The research group of Maureen Hanson measured various molecules in urine samples before and 24 hours after a cardiopulmonary exercise test (CPET). Only 10 ME/CFS patients were included in this study, but each urine sample was screened for 1403 metabolites, much more than in previous studies.
Before exercise, no significant differences were detected between controls and ME/CFS patients. After exercise, however, four compounds were significantly different, all of which were found at lower concentrations in the ME/CFS patients compared to the healthy controls. As the authors note: “Our most unanticipated discovery is the lack of changes in the urine metabolome of ME/CFS patients during recovery while significant changes are induced in controls after CPET, potentially demonstrating the lack of adaptation to a severe stress in ME/CFS patients.”

Endothelial dysfunction
Endothelial dysfunction and tissue hypoxia have emerged as prominent theories in both ME/CFS and Long Covid. The endothelium, found on the inner lining of blood vessels, plays a crucial role in regulating blood flow. Dysfunction of the endothelium therefore can lead to inadequate oxygen supply to muscle tissue, affecting its ability to meet energy demands.
A Norwegian group has been pursuing this hypothesis for a couple of years. In 2021, they published evidence of endothelial dysfunction in ME/CFS patients who participated in the cyclophosphamide trial. This year they were able to replicate these findings in participants of the Rituximab trial.
The testing went as follows. First, a cuff was placed around the participants’ forearm to temporarily block blood flow. When they released the cuff to restore blood flow, the researchers measured the artery diameter and change in blood flow using ultrasound imaging. Both these measures were reduced in ME/CFS patients compared to healthy controls, providing objective evidence of endothelial dysfunction.
There was only one problem. These abnormalities did not correlate well with measures of illness severity such as physical functioning, or the number of steps patients made. It therefore remains unclear if these measurements of endothelial function also have clinical value in ME/CFS.
microRNAs
2023 also saw two replication attempts in the study of microRNAs (miRNAs); the small non-coding RNAs that regulate gene expression.
The Canadian research team of Alain Moreau tested 11 circulating miRNAs that were associated with ME/CFS in previous studies. 3 of these miRNAs were significantly higher in ME/CFS patients compared to healthy controls. Moreau and his team also tested the miRNAs in patients with fibromyalgia and found that they were significantly reduced compared to both ME/CFS patients and healthy controls. These opposite results for ME/CFS (increased values) and fibromyalgia (reduced values) are interesting, but they could also be the result of different sampling methods: ME/CFS patients were recruited directly into the study, while the fibromyalgia samples came from the CARTaGENE biobank.
An Italian-Lithuanian study also tested 8 miRNAs that have been associated with ME/CFS or with various auto-immune diseases. In their cohort of 40 ME/CFS patients, 3 of the measured miRNAs were upregulated compared to controls, confirming previous data. The largest difference was found for miR-448 which had not been tested in ME/CFS before but was found to be increased in auto-immune diseases such as rheumatoid arthritis, Sjögren’s, and Lupus.
Brain scans
There were also a couple of ME/CFS imaging studies in 2023.
The most interesting one came from the Australian team of Leighton Barnden at Griffith University. They used one of the most powerful MRI scans in the world with an ultra-high field strength of 7 Tesla. Barnden and colleagues found that the brainstem region was enlarged in both ME/CFS and Long Covid patients by about 10 to 20 percent compared to controls. Unfortunately, the sample sizes were small: only 10 ME/CFS and 8 Long Covid patients were studied. Nonetheless, this is an exciting result that will hopefully be studied further in 2024.
There were also two imaging studies from another Australian research group that focused on adolescents with ME/CFS. One of the studies zoomed in on the hypothalamus, while the other used Diffusion MRI a technique that uses diffusion of water molecules to generate contrast in MR images. Unfortunately, both studies did not find any clear abnormality.
Long Covid and other illnesses
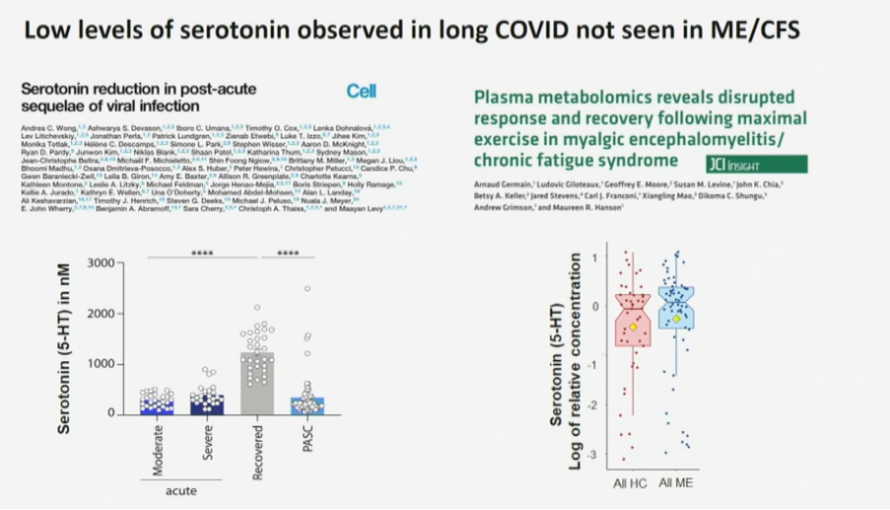
In the field of Long Covid, a large collaboration of researchers reported a depletion of serotonin in patients compared to controls. The researchers were able to develop a mice model that showed similarly decreased plasma serotonin levels when the mice were infected by the vesicular stomatitis virus. The authors also note that lower serotonin levels have been found in other settings of viral inflammation, such as dengue virus infection. They suspect that serotonin depletion disrupts the activity of the vagus nerve and that this might be an explanation for the cognitive problems of Long Covid patients.
The large majority of circulating serotonin is produced in the gut, where it is synthesized from tryptophan that we extract from our diet. The researchers were able to show that tryptophan levels were also decreased in Long Covid patients.
The paper consists of an impressive series of experiments, but the explanation almost seems too simple. Serotonin is a neurotransmitter that has been studied a lot and it would be quite a surprise if nobody has picked up on this before. Previous ME/CFS studies did not find serotonin levels to be reduced in patients and selective serotonin reuptake inhibitors (SSRIs) have failed to improve ME/CFS symptoms. Something similar can be said about the reduced cortisol levels that have been reported in Long Covid patients: in ME/CFS results on cortisol have been conflicting, to say the least. Perhaps these markers are only abnormal shortly after the infection that triggers the syndrome.
In fibromyalgia research, there was a decent trial of Low-Dose Naltrexone (LDN) that unfortunately did not find a significant benefit in relieving pain. This is important because some physicians prescribe LDN to ME/CFS patients believing it has been shown to work in fibromyalgia. The Open Medicine Foundation is currently planning a randomized trial of LDN in ME/CFS patients that might provide more information on its usefulness.
Lastly, a large, randomized trial published in the Lancet tested low-dose oral amitriptyline for irritable bowel syndrome (IBS, just like fibromyalgia a frequent comorbidity of ME/CFS). The tricyclic antidepressant had a beneficial effect on IBS symptoms but unfortunately, the effect was rather small.
Honorable mentions
We end our review with two honorable mentions.
Home-based testing
The first study implemented a home-based testing protocol to measure physiological responses to everyday activities in ME/CFS. The physiotherapists of Physios for ME visited the homes of 17 ME/CFS patients with their portable metabolic assessment devices. Blood pressure, heart rate, oxygen saturation, and lactic acid were assessed during a range of everyday positions and activities. Examples were sitting for 5 minutes, preparing breakfast, washing yourself, walking upstairs, etc. The researchers found that ME/CFS patients often exceeded their anaerobic threshold during these everyday activities.
Although this is just a feasibility study, it could provide a new framework for testing ME/CFS patients during PEM. Current studies often focus on short and intense exercises such as a bicycle test to provoke PEM which may lead to misleading results. For example, some ME/CFS patients have indicated that the trip to the doctor already caused PEM before they were on the exercise bike. Measuring physiological responses in patients’ homes after everyday activities may lead to a more accurate assessment of PEM.
The FUNCAP questionnaire
The other honorable mention is the FUNCAP questionnaire developed by Kristian Sommerfelt and Trude Schei of the Norwegian ME Association. It consists of 55 questions to assess the functional capacity of ME/CFS patients, for example, for disability benefits or insurance purposes. It asks about various day-to-day tasks such as ‘showering standing up’, ‘having a conversation for approximately 5 minutes’, ‘going to a shop for groceries’, ‘using public transport’ etc. There is also a shorter version with 27 questions for international research. Both versions are available for free here (see the supplementary material).
The great thing about FUNCAP is that it was developed with strong patient consultation (there were multiple survey rounds where respondents could provide feedback) and that it takes PEM into account. Other functional assessments usually present a list of activities or tasks and expect a yes or no answer: can you do this or not? For ME/CFS patients, the correct answer is often more complex. They might be able to overexert themselves and do the activity once but will then become sicker, forcing them to reduce their other activities. FUNCAP therefore presents you with 7 options. For each suggested activity you can answer:
- 0: I cannot do this.
- 1: My capacity will be severely reduced for at least three days.
- 2: I can do little else on the same day and for one or two days afterward.
- 3: I can do little else on the same day.
- 4: I must limit other activities on the same day.
- 5: This rarely affects other activities.
- 6: Unproblematic – does not affect other activities.
Hopefully, this FUNCAP questionnaire will be further tested and used by ME/CFS researchers.
That’s a wrap! If you missed any important ME/CFS study that we omitted in our review, feel free to post them in the comment section below.
Big thanks to everyone at the Science for ME forum whose thoughtful discussions and detailed analyses of papers have helped us immensely in creating this yearly review.
Happy Holidays and a great 2024 to all!
Great overview! It’s hard to keep track of what all is being looked at through the year, so happy to have this!
The Hwang research seems significant. I’m almost afraid to start hoping.